HOME RESEARCH PUBLICATION MEMBER RECRUITMENT GALLERY LINK CONTACT
The organs of the body all play critical functional roles, which are made possible by the arrangement of differentiated cells into structures specific to that organ. Such structures are formed through the process of development, with the late embryonic and immediate postnatal periods being particularly important for the functional maturation of organ systems. Defects that arise during these organogenetic processes are closely linked to a wide range of diseases. Postnatally, the body is also constantly exposed to environmental stresses that may be potentially damaging to organ systems. While the adult body does display a certain degree of regenerative abilities in response to injury or damage, this is by no means complete. To study organ formation, repair and regeneration, we have focused our research on the respiratory system in the mouse model.
Respiratory organs in higher mammals are characterized by highly efficient gas exchange, which is enabled by the function of specialized cells. The formation and development of respiratory organs are orchestrated by complex mesenchymal-epithelial interactions that coordinate the temporal and spatial expression of multiple regulatory factors required for proper organ formation (Fig.1). The conducting airways including the trachea, bronchi, and bronchioles play a role in delivering external air into the alveoli and prevent invasion of foreign enemies. The function of the conducting airways is maintained by physiological function of five types of epithelial cells—club cells (secretory cells), ciliated cells, neuroendocrine cells, basal cells, and goblet cells—and the numerical balance of these cells (Fig. 1). In the adult trachea and lungs, the epithelium pushes out the dust and bacteria that enters the airway from the external environment, through a process known as mucociliary clearance, which is dependent on the differentiation and distribution of goblet cells, club cells and ciliated cells. The alveoli are the main body of gas exchange in the lungs, showing an elaborate, elastic sponge-like tissue structure, which facilitates efficient gas exchange. Alveolar epithelial type I cells are located adjacent to the flat capillary, where gas exchange takes place. Alveolar epithelial type II cells are cuboidal in shape and secrete surfactants to maintain alveolar tissue structure. Interestingly, all cell types can be produced from common fetal epithelial stem cells.
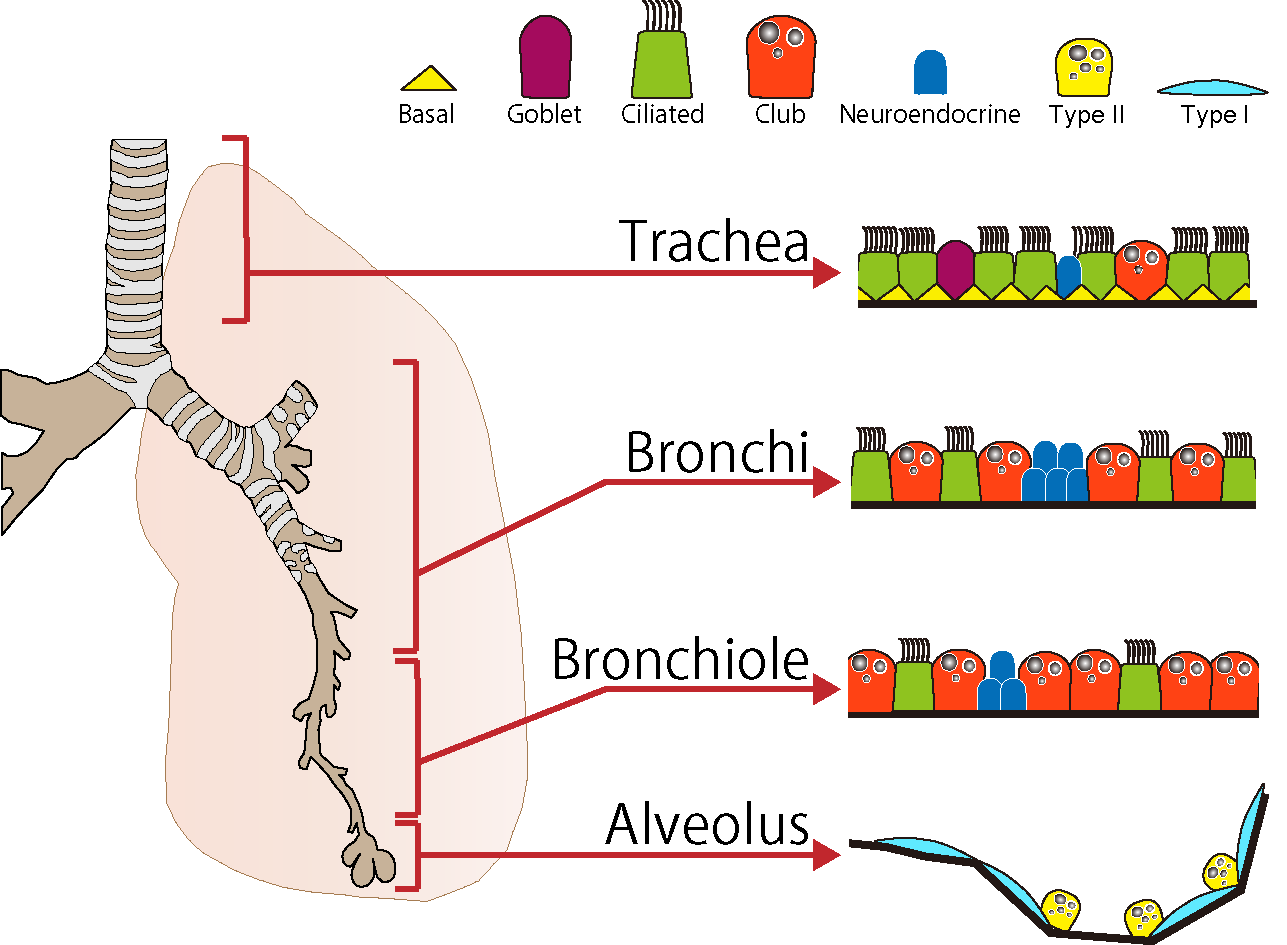
<Fig.1>The epithelial cell types in the conducting airways and alveoli of mouse
In our work to date, we have reported that the number, distribution and function of nearly all epithelial cell types of the trachea and lungs are affected by Notch signaling (Fig. 2). We also succeeded in carrying out live-imaging of migrating pulmonary neuroendocrine cells in developing fetal lungs for the first time in the world by combining cutting-edge high resolution four-dimensional imaging technology and fetal lung organ culture technique. Our work on comprehensive and quantitative analyses of developing trachea, which is a large tubular tissue, revealed that polarity synchronization of mesenchymal cells and cell differentiation are timed precisely to control the length and diameter of the trachea. These findings show that while tissue structure and function of the respiratory system is complex, its developmental processes are accurately programmed by genes. Intercellular signals observed during fetal development have also been found to occur in adult regenerating tissue as well as in diseases.
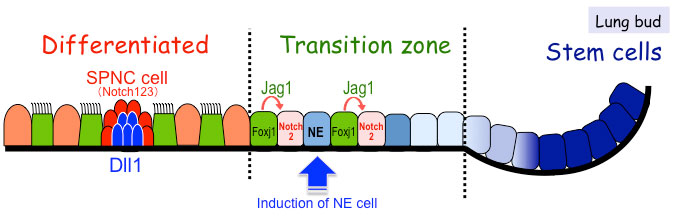
<Fig.2>Schematic diagrams of the proposed regulatory mechanisms involved in
Notch-dependent cell fate determination during lung epithelial development
Late embryonic to early postnatal stages are important periods for establishing organ functions and are also critical stages for understanding the onset of diseases due to developmental abnormalities. It is also known that there are some common points between organ formation in the later stage of development and tissue regeneration in adults. In this project, we analyze single-cell transcriptome and individual cell kinetics of the developing epithelium to elucidate the cooperative mechanism of multicellular system to establish the mature organ in late developmental stages. We also search for key factors involved in maintaining multipotency of stem cells in the embryo and cancer in adults.
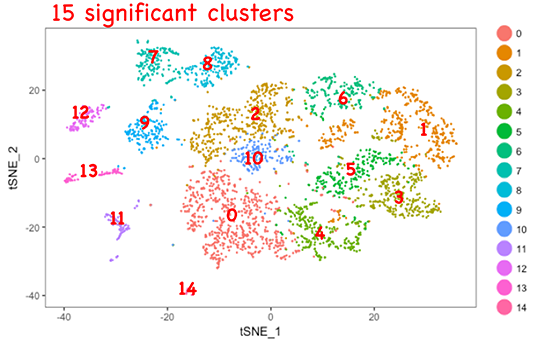
<Fig.3>Analysis of single-cell transcriptome in fetal epithelium.
In addition to gas exchange, there are two important functions of the airways. One is to detect the respiratory environment, and the second is to respond to a tissue injury caused by invasion of external factors, such as pollutants and viruses. NE cells play an important role in both of the functions, and clusters of NE cells are known as "respiratory sensory organs". We successfully carried out live-imaging of fetal NE cells, and revealed that NE cells undergo directional migration and accumulate at the bronchial branch points. In this project, we are studying the mechanism of migration of NE cells and the relationship between NE cell function and diseases in the adult.
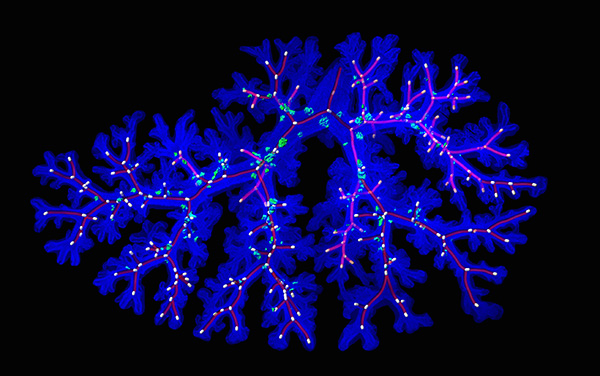
<Fig 4> Distribution pattern of pulmonary NE cell (green) on developing bronchi (blue). Red lines show bifurcation structure of bronchi.
The lungs become functional when a baby begins breathing in air immediately after birth. The onset of respiration places mechanical stress on the lung tissue and exposes it to high concentrations of oxygen. These two modes of stimuli are thought to promote further development of the lung tissue. Our previous studies have shown that the Notch2 signal is essential for alveolar formation in newborn mice, and that a loss of Notch-related genes cause bronchopulmonary dysplasia (BPD), leading to emphysema. Other intercellular signals, such as Wnt and Yap signaling, have also been reported to regulate postnatal alveolar development, and defects in this signaling pathway resulted in a BPD- like phenotype, suggesting that complex cell-cell signal crosstalk is required in postnatal alveolar development. In this project, we aim to elucidate the mechanisms by which respiratory initiation stimulates alveolar tissue stem cells by using mutant mice showing abnormalities in alveolar formation after birth.
<Fig 5> Histological comparison of the lungs in wildtype and in Notch-deficient (Pofut1 mutant) mice. Whereas no significant differences were observed in embryonic lungs, after birth, the lungs in the mutant mouse displayed abnormal alveologenesis, with airspaces within tissue becoming enlarged and the tissue becoming less dense (left panels). Lungs in mutants had fewer numbers of proliferating type II cells (light blue and arrowheads) (far right panels).
Project 4: Reconstructive understanding of respiratory system.
Stem cell culturing technology has seen remarkable advances over the past several years, and has become a driving force for life-science research. In particular, "organoid culture", which is a technique involving the reconstruction of mini-organs from stem cells, has recently drawn much attention. We are working to develop technology for reconstructing and culturing a part of the respiratory tract from stem cells, using engineering and chemistry approaches as well as microdevices. By generating a respiratory organoid, we expect to be able to observe the stem cells dynamics in a relatively simple experimental system, to demonstrate proof of concept, and to develop an in vitro pathology model.